На главную ✨ Ана Михалча ✨ Мик Андерсен ✨ Клиффорд Карником ✨ Брайан Ардис
Ana Maria Mihalcea, MD, PhD - Dec 15, 2023 ∙ Paid ∙ Source
Likes: 116 | Comments: 74 | Reposts: 19 | ALL OTHER POSTS
Image: Micellar and self assembly fibrillar Amyloid proteins for nanotechnology applications
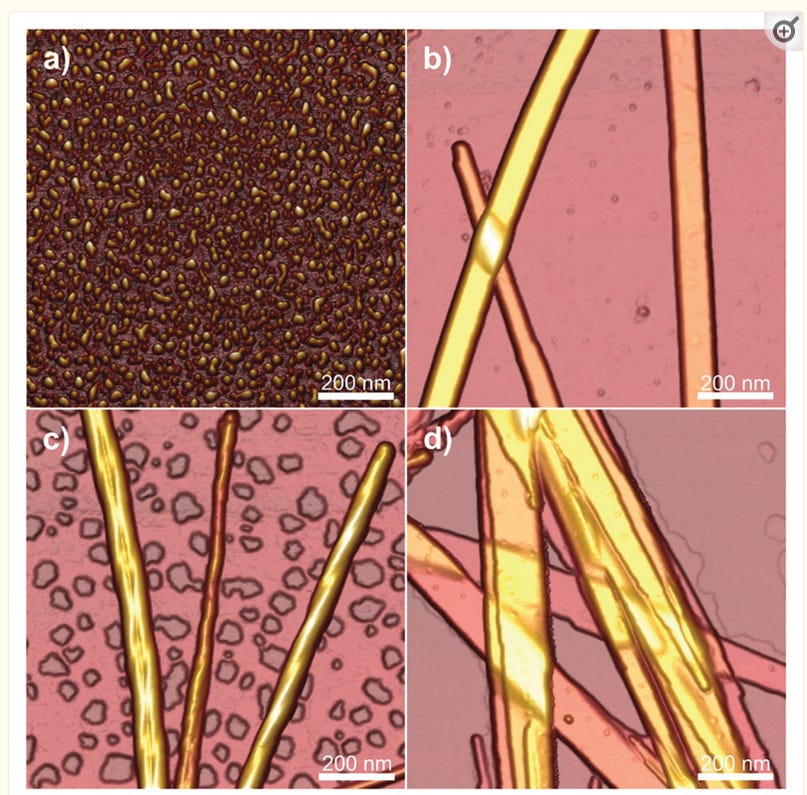
Tapping mode AFM height images showing the morphology of C16-KTTKS at pH values of (a) pH 2, (b) pH 3, (c) pH 4, (d) pH 7. Reprinted with permission from ref. 76 . Copyright 2013, Royal Society of Chemistry.
For over a year now, I have been trying to educate people that its nanotechnology hydrogels we are dealing with from the C19 bioweapons, not Amyloid. This technological weapon was also what “Covid” was, a gain of function created biotechnological synthetic biology nano weapon that is producing hydrogel biosensing materials. Yes, this most recent Russian study confirms what we have been saying all along, but they still do not understand that the Amyloid is used for Nanotechnology bio sensing applications. Russian Study Finds Self Assembly Nanoparticles and Nanofibers: "Self-assembling amyloid-like nanostructures from SARS-CoV-2 S1, S2, RBD and N recombinant proteins"
The difference is important, because it affects what these protein polymers are used for and what threats we should be aware of. The medical community can continue to deny Nanotechnology if we use Amyloid or Amyloid like, and keep denying our findings. But the science my friends, is clear. It is all Nanotechnology building blocks as I will explain below.
Here is the article I wrote last year, reviewing an article that clearly said that at pH of 4 the spike protein sequence produced Amyloid, but at pH 7 which is where we are is produced Hydrogel.
Here is is discussed how amyloid peptides are used to create hydrogels:
Dynamics of the formation of a hydrogel by a pathogenic amyloid peptide: islet amyloid polypeptide
Here is a review of the use of amyloids in Nanotechnology:
Self-assembling peptide and protein amyloids: from structure to tailored function in nanotechnology
Self-assembled peptide and protein amyloid nanostructures have traditionally been considered only as pathological aggregates implicated in human neurodegenerative diseases. In more recent times, these nanostructures have found interesting applications as advanced materials in biomedicine, tissue engineering, renewable energy, environmental science, nanotechnology and material science, to name only a few fields. In all these applications, the final function depends on: (i) t he specific mechanisms of protein aggregation, (ii) the hierarchical structure of the protein and peptide amyloids from the atomistic to mesoscopic length scales and (iii) the physical properties of the amyloids in the context of their surrounding environment (biological or artificial). In this review, we will discuss recent progress made in the field of functional and artificial amyloids and highlight connections between protein/peptide folding, unfolding and aggregation mechanisms, with the resulting amyloid structure and functionality. We also highlight current advances in the design and synthesis of amyloid-based biological and functional materials and identify new potential fields in which amyloid-based structures promise new breakthroughs.
Specifically, please note that the amyloid fibrils are used as nanomaterial building blocks for biosensors and nanodevices as well as synthetic biology applications like the fusion of humans with technology by creating biomembranes. Energy conversion materials also is translated into energy harvesting or hijacking your life force.
In addition to toxic and functional amyloids, in recent years there has been a growing interest in the applications of amyloid fibrils as templates or building blocks in ordered nanomaterials for biomedical, biomaterial and nanotechnological applications. Amyloid nanofibrils have been successfully employed as a fundamental component in biomembranes, functional nanodevices, hydrogels for cell culture and drug delivery, biosensors, functional materials with high biocompatibility and unique bio-recognition ability and as energy conversion materials.
Please read carefully here that amyloid creates all that we have been finding but it is part of the nanotechnology application - it is used because it is “biocompatible”. That of course is a joke, because you die of self assembly clots. I knew someone who died in a few months from systemic unexplained “amyloidosis” from C19 Bioweapon shedding - some people cannot clear this poison and it grows like a cancer. In reality it was death by self assembly nanotechnology.
In sharp contrast, the mesoscopic structure of amyloid fibrils shows a remarkable diversity, with a multitude of shapes and topologies, depending on the specific aggregation pathways followed. To date, nanoparticles, nanofibrils, nanotubes, ribbons, nanosheets and 3D scaffolds or multilayers represent just some of the amyloid morphologies observed. A wide spectrum of available morphologies and free energies, high surface-to-volume ratio, high density of hydrogen bonds and the presence of biocompatible amino acids on their surfaces gives amyloid fibrils a remarkable range of nanomechanical properties and applications across many scientific fields.
In the article they describe that according to pH, either micellar structures or fibrils develop.
This is exactly what I have been seeing in the live blood analysis of C19 unvaccinated individuals exposed to C19 bioweapon shedding.
On the right you can see the classic micellar areas that use the blood as a substrate and transform it into rubbery clot material. I see this in everyone’s blood now. It is the structures from which the filaments grow. On the left is the classic filament structures, surrounded by the classic micro robotic biosensors. I have shown before how the construction process works in the blood. Please see the image of micellar and fibril amyloid above as a comparison.
Image: C19 unvaccinated blood contaminated with Nanotechnology via C19 shedding and environmental exposure
I have also shown the rubbery clot formation. As we see below, polymerization of Amyloid nanotechnology is temperature dependent. Rubbery Clot Formation Shown In Living C19 Unvaccinated Person Via Darkfield Microscopy - Hydrogel Replacing Blood Rubbery Clot Formation Shown In Living C19 Unvaccinated Person Via Darkfield Microscopy - Hydrogel Replacing Blood
This is what is discussed in the nanotechnology literature about amyloid peptide formation. You cannot cool down the core body temperature, so you cannot use cold denaturation in humans. I agree in my observations in the blood that there are changes in density of the material from gel like to a more denser fibril form.
Heating (with or without pH adjustment to acidic conditions) is another common method of inducing amyloid formation with proteins and peptides. Again, there are too many studies involving heat treatment to review them all and space permits only selected examples to be discussed herein. At sufficiently high concentration, the fibrillization of peptides is accompanied by gelation. For example, β-lactoglobulin forms fibrillar gels on heating at low pH values. Particulate gels are formed at higher pH values, close to the isoelectric point where the protein has a low net charge. Cold denaturation is generally a milder form than hot denaturation and leads only to a partial unfolding of proteins and so, rarely, amyloid formation is reported under these conditions. However, cold denaturation can be used to dissociate amyloid fibrils, as discussed below.
Amyloid, Prion is all the same thing, dependent on how the protein are folded and what kind of metal ions are used. When I have been explaining of how important it is to detoxify metals as they become the semiconducting antenna of nano biosensors, but it is also important because metals act as a catalyst that make these polymer aggregates grow faster and bind together stronger.
Apart from Aβ, di- and tri-valent metal ions have been shown to cause significant increases in the rate of fibril formation of α-synuclein and there appears to be a correlation to the ion charge density.
Nanoparticle metals accelerate the fibril growth:
Gold and silver nanoparticles have been shown to accelerate fibril growth of the NNFGAIL peptide from human islet amyloid polypeptide and the prion protein Sup35 peptide GNNQQNY in physiological aqueous solutions.
Both metal ions and metal nanoparticles could promote the formation of amyloid fibrils, but the nature of their binding with proteins is different. Metal nanoparticles adhere with amyloids predominantly through electrostatic interactions and surface tension reduction, whereas metal ions bind to amyloids via supramolecular metal–ligand interactions.
Below the relationship between amyloid nanostructures and prions are described. These amyloid nanoparticles ARE INFECTIOUS AKA SHEDDING AND HIGHLY CYTOTOXIC CAUSE FOR PRION DISEASE. Note these are man made nanotechnology building blocks.
Prefibrillar amyloid structures, such as spheroidal aggregates (nanoparticles and nanospheres), similar to the molecular oligomers introduced previously, have been frequently proposed to be a highly cytotoxic species in many neurodegenerative diseases. Silveira and co-workers degraded large prion protein (PrP) aggregates into smaller PrP nanoparticles with sizes ranging from 17 to 27 nm, and then characterized the PrP nanoparticles with DLS, non-denaturing gel electrophoresis and transmission electron microscopy (TEM). Their findings suggested that the PrP nanoparticles with masses equivalent to 14–28 PrP molecules are the most infectious initiators for prion diseases. In another study, EI Moustaine et al. formed amyloid nanofibrils and nanoparticles from recombinant PrP at high pressure.
Twisted and helical amyloid ribbons: (a) left-handed β-lactoglobulin nanofibrils with multistranded twisted filaments. Reprinted with permission from ref. 233 . Copyright 2010, Nature Publisher. (b) Cross-β amyloid TTR 105–115 fibril with triplet atomic-resolution structure. Reprinted with permission from ref. 294 . Copyright 2013, National Academy of Sciences. (c) Twisted right-handed helical ILQINS hexapeptide ribbon. Reproduced with permission from ref. 297 . Copyright 2014, American Chemical Society. (d) Twisted double-helical peptide ribbon, Reprinted with permission from ref. 298 . Copyright 2009, Wiley-VCH Verlag GmbH & Co. (e) Amyloid-inspired rippled β-sheet ribbons by the co-assembly of enantiomeric amphipathic peptides. Reprinted with permission from ref. 299 . Copyright 2012, American Chemical Society.
Here again same thing we have been observing in Live Blood has been done in experiments:
These amyloid nanofibrils have been combined with Carbon Nanotubes ( aka Graphene Oxide)
Here is what the amyloid fibrils look like when combined with polystyrene, silica and Gold. Remember all those microplastics that were found in cardiac tissue including polystyrene?
CR and NBT assays confirm the amyloidogenic features and the formation of an amyloid–DOPA hybrid. (b) TEM images of unmodified and modified amyloid nanofibrils with biomolecules. (c) Schematic presentation of the measurement of the adhesion force between amyloid nanofibrils and biomolecules with AFM force spectroscopy. Representative AFM image showing modified Mfp5-CsgA fibres 1 h after deposition on a mica surface. Reproduced with permission from ref. 394 . Copyright 2014, Nature Publishing Group.
There are plenty of publications that explain Prion Amyloids for Nano-technological bio sensing applications:
Functionalized Prion-Inspired Amyloids for Biosensor Applications
Prion-inspired heptapeptides were doped with biotin-conjugated counterparts and assembled into amyloid-like fibers under mild conditions. The scaffolds’ versatile functionalization was demonstrated by decorating them with different streptavidin conjugates, including gold nanoparticles, quantum dots, and enzymes.
An auto-biotinylated bifunctional protein nanowire for ultra-sensitive molecular biosensing
In order to obtain an ultra-sensitive molecular biosensor, we designed an auto-biotinylated bifunctional protein nanowire (bFPNw) based on the self-assembly of a yeast amyloid protein ,
Amyloid-based nanosensors and nanodevices
Recent studies have highlighted the use of self-assembled amyloid-based fibers as nanomaterials.
Likes: 116 | Comments: 74 | Reposts: 19 | Share Options: Copy Link
СОВЕТ: Чтобы быть в курсе всех обновлений, а также находок микроскопии и способов детокса нанотехнологий в российских условиях, подписывайтесь на телеграм-канал Нанороботы в крови☣️Микроскопия👀Наука🌍Детокс🌞.
На главную ✨ Ана Михалча ✨ Мик Андерсен ✨ Клиффорд Карником ✨ Брайан Ардис
Marianne Agnello - Dec 15, 2023
Marianne Agnello
Thank you Ana
That this has been going on fir so many years it shows how the medical profession has been duped with no-one waking up to the possibility of it being used as a weapon on people with the result the medical profession who have been on a pedestal for so long were quite willing to be paid BIG BUCKS to inject everyone regardless of their health status
Time to wake up and don't comply
REPLY | 7 replies
Margie Chism - Dec 16, 2023
Margie Chism
What exactly is this vital “life force?” ■
It’s called glutathione (glue-ta-thigh-own) and it’s the mother of all antioxidants—5,000 times stronger than any other antioxidant in your body.
In fact, scientists consider glutathione the single most important factor in aging!
Every cell in your body makes its own supply of glutathione as do your tissues, and organs10.
Just like you must have air to breathe, your cells and organs must make glutathione to maintain healthy function… and defend against things like free radicals and toxins that seek to destroy your health.
Don’t discount this for one second…
These invaders are like heat seeking missiles determined to extinguish your life force. That’s why I call them “life force liquidators.” More on those destroyers shortly…
■https://www.greenvalleynaturals.com/landers/VIFO/VIFO121123a?AFFID=bhb_solo&C1=121523&C2=super_agers&C3=vifo&utm_source=email&utm_medium=solo&utm_campaign=vifo&utm_content=this_is_why_youre_always_tired&utm_term=bhb_solo_121523
https://www.greenvalleynaturals.com/landers/VIFO/VIFO121123a?AFFID=bhb_solo&C1=121523&C2=super_agers&C3=vifo&utm_source=email&utm_medium=solo&utm_campaign=vifo&utm_content=this_is_why_youre_always_tired&utm_term=bhb_solo_121523
REPLY | 7 replies
72 more comments...
anaunited anapost